Learning Outcomes
Students will be able to:
i. Define the first law of thermodynamics and explain its significance in understanding energy conservation.
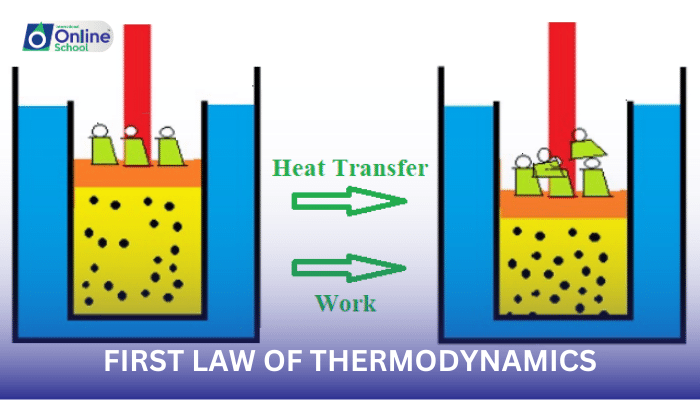
ii. Express the first law of thermodynamics mathematically as ΔU = Q + W, where ΔU represents the change in internal energy, Q indicates the heat absorbed by the system, and W represents the work done on the system.
iii. Apply the first law of thermodynamics to analyze energy transformations in various phenomena.
iv. Appreciate the limitations of the first law of thermodynamics and its applicability to ideal systems.
Introduction
In the grand orchestra of nature, energy plays a pivotal role, powering our existence and shaping the world around us. The first law of thermodynamics, a cornerstone of physics, asserts that energy is never created or destroyed; it can only be transformed from one form to another. This fundamental principle governs the behavior of energy in various systems and processes.
i. Energy Conservation: A Symphony of Transformations
Imagine a closed system, such as a container of gas. The total energy of this system remains constant, even though energy can be transformed between different forms. For instance, when the gas is heated, its internal energy increases, representing a transformation from heat energy to internal energy.
The first law of thermodynamics provides a mathematical framework for understanding these energy transformations. It states that the change in internal energy of a system (ΔU) is equal to the sum of heat absorbed by the system (Q) and the work done on the system (W):
ΔU = Q + W
This equation encapsulates the essence of energy conservation, indicating that the total energy of an isolated system remains constant.
ii. Analyzing Energy Transformations: The First Law in Action
The first law of thermodynamics finds applications in various phenomena:
Heating and Cooling: When a substance is heated, its internal energy increases, reflecting the absorption of heat. Conversely, when a substance cools, its internal energy decreases, indicating the release of heat. The first law provides a quantitative measure of these energy transfers.
Phase Changes: Phase changes, such as melting, evaporation, and condensation, involve transitions between different states of matter and are accompanied by changes in internal energy. The first law helps us analyze the energy transfers associated with these transitions.
Engine Operation: Engines, such as internal combustion engines, rely on energy transformations governed by the first law. The combustion of fuel releases heat, which is then converted into mechanical work through a series of energy transformations.
iii. Limitations and Ideal Systems: A Symphony with Nuances
While the first law of thermodynamics is a powerful tool for understanding energy transformations, it has certain limitations:
Irreversibility: Many natural processes are irreversible, meaning they cannot be completely reversed without external intervention. The first law does not account for irreversibility, which limits its applicability to certain situations.
Ideal Systems: The first law is most applicable to ideal systems, which are theoretical constructs that do not exist in the real world. Real systems often involve energy losses due to friction, heat transfer to the surroundings, and other factors that deviate from the ideal conditions.
The first law of thermodynamics, a fundamental principle in physics, provides a framework for understanding energy conservation and transformations. Its applications extend far beyond the realm of physics, shaping our perception of the world and enabling us to harness the power of energy in countless ways. As we continue to explore the universe, the first law remains a guiding principle, illuminating the path to new discoveries and technological advancements. Its influence extends to various fields, from engineering and chemistry to biology and beyond, shaping our understanding of the intricate dance of energy in the grand symphony of nature.